Details of the Drug
General Information of Drug (ID: DMSZFTP)
| Drug Name |
Met-enkephalin
|
||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Synonyms |
MET-enkephalin; H-Tyr-Gly-Gly-Phe-Met-OH; 58569-55-4; Tyr-Gly-Gly-Phe-Met-OH; METENKEFALIN; [Met]enkephalin; [5-Methionine]Enkephalin; TYR-GLY-GLY-PHE-MET; Enkephalin M; UNII-9JEZ9OD3AS; (Met5)-enkephalin; Lupex; 9JEZ9OD3AS; CHEMBL13786; CHEBI:6618; ENKEPHALIN, METHIONINE; Opioid growth factor; [Met5]-ENKEPHALIN; [Met5]Enkephalin acetate salt hydrate; Porcine beta-endorphin 1-5; (2S,5S,14S)-14-Amino-5-benzyl-15-(4-hydroxyphenyl)-2-(2-(methylthio)ethyl)-4,7,10,13-tetraoxo-3,6,9,12-tetraazapentadecan-1-oic
|
||||||||||||||||||||||
| Indication |
|
||||||||||||||||||||||
| Drug Type |
Small molecular drug
|
||||||||||||||||||||||
| Structure |
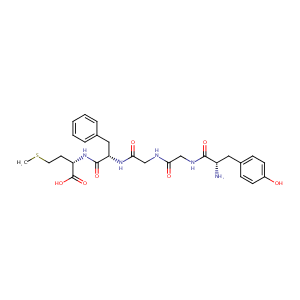 |
||||||||||||||||||||||
| 3D MOL | 2D MOL | ||||||||||||||||||||||
| #Ro5 Violations (Lipinski): 4 | Molecular Weight (mw) | 573.7 | |||||||||||||||||||||
| Logarithm of the Partition Coefficient (xlogp) | -2.1 | ||||||||||||||||||||||
| Rotatable Bond Count (rotbonds) | 16 | ||||||||||||||||||||||
| Hydrogen Bond Donor Count (hbonddonor) | 7 | ||||||||||||||||||||||
| Hydrogen Bond Acceptor Count (hbondacc) | 9 | ||||||||||||||||||||||
| ADMET Property |
|
||||||||||||||||||||||
| Chemical Identifiers |
|
||||||||||||||||||||||
| Cross-matching ID | |||||||||||||||||||||||
Molecular Interaction Atlas of This Drug
 Drug Therapeutic Target (DTT) |
|
|||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular Interaction Atlas (MIA) | ||||||||||||||||||||||||||||||||
Molecular Expression Atlas of This Drug
| The Studied Disease | Pain | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ICD Disease Classification | MG30-MG3Z | |||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Molecular Expression Atlas (MEA) | ||||||||||||||||||||||||||||||
References
